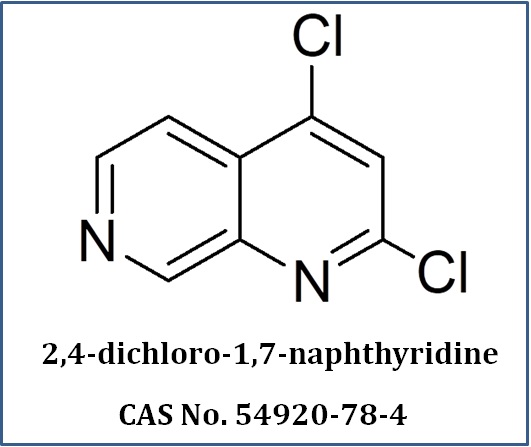
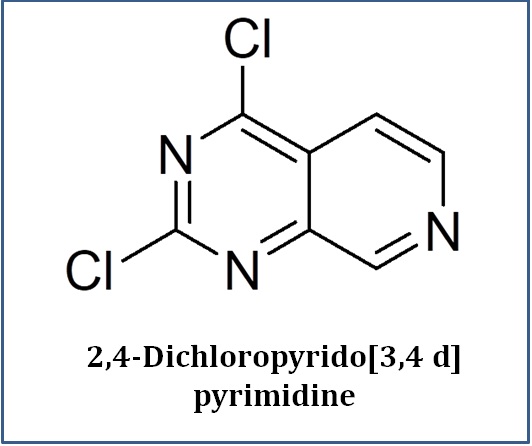
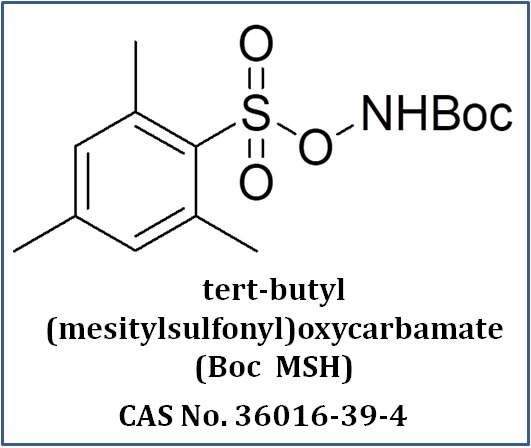
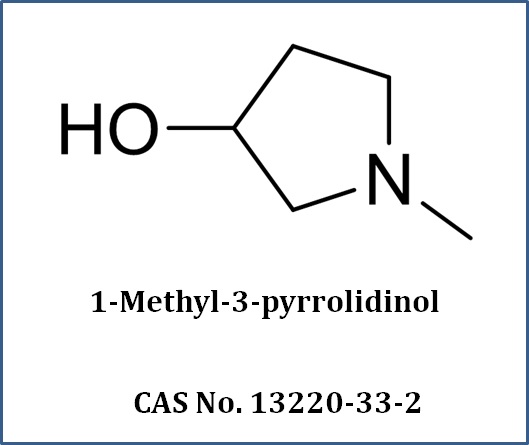
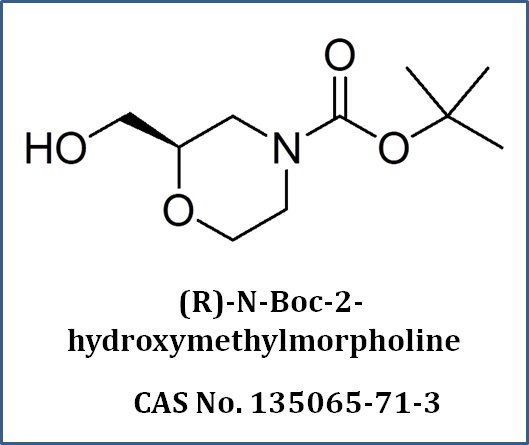
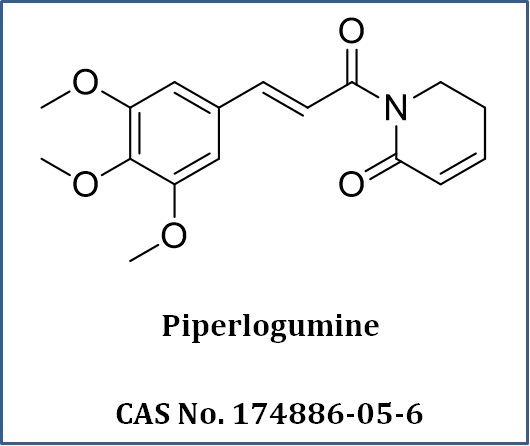
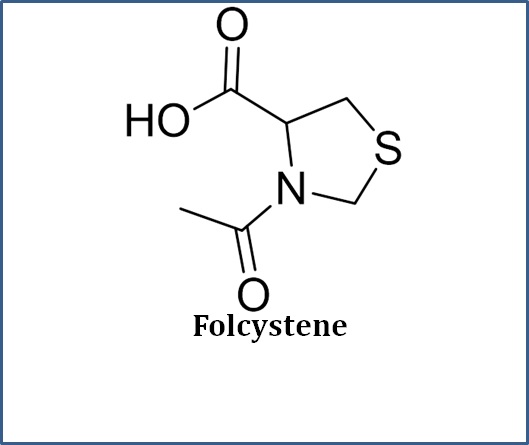
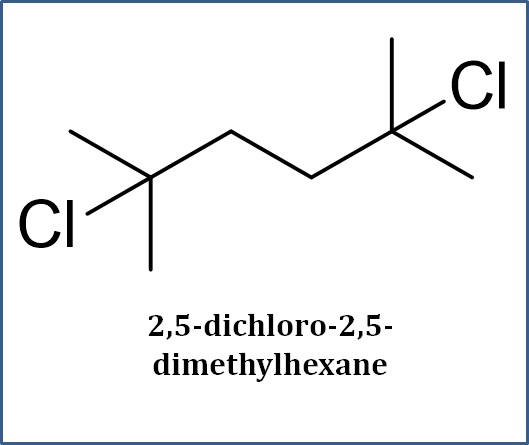
What We Do
ICH guidelines for Drug Substances and Drug Products require that related impurities and degradants in Active Pharmaceutical Ingredients (APIs) and Drug Products be structurally identified at certain threshold levels depending on dose.
Our capabilities in
- Synthesis of impurities in mg to gm scale
- Isolation / purification of impurities using chromatographic techniques
- Characterisation / identification of impurities by HPLC, LCMS, GCMS, NMR, MS and IR
“Our knowledge of chemistry gives us an edge in identifying even the most difficult API impurity and degradant isolation and identification solutions”